Research
How does the brain sense protein availability to control appetite and metabolism?
Protein is the macronutrient that has the strongest influence on appetite, but how the brain represents protein availability to regulate hunger and satiety is poorly understood.
Compelling evidence indicates that the control of protein intake is prioritised over the control of energy intake, leading to changes in energy balance. Eating a high protein diet can be detrimental to health on the long-term. However, specifically targeting brain protein sensing mechanisms may represent a successful strategy to produce satiety in the absence of compensatory changes in energy expenditure, leading to weight loss.
One of our ongoing interest is to characterise brain protein sensing mechanisms involved in the control of appetite in rodents.
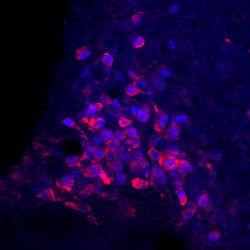
Recently, we have shown that neurons of the nucleus of the solitari tract (NTS) expressing Calcitonin Receptor (CalR) and Prolactin Releasing Hormone (Prlrh) sense the amino acid Leucine, a signal for body's protein availability. We found that these leucine-sensing neurons have unique properties , making them excellent candidates for appetite-suppressive drugs.
First, unlike many of appetite-regulating neurons in the NTS, activation of CalR/Prlrh neurons do not produce aversion (Cheng W, Cell Metabolism, 2020). This is important because nausea is one of the most common side-effect of current anti-obesity drugs.
Second, they project to and inhibit Agrp neurons in the hypothalamus, thus directly reducing hunger (Tsang A, Molecular metabolism, 2020), leading to negative energy balance.
Current projects in the lab continue to characterise the molecular and neuroanatomical properties of distributed protein sensing sites and their roles in distinct behavioural, metabolic and neuroendocrine functions regulated by dietary proteins.
Mechanisms underpinning the synergistic weight and feeding suppressive effects of drug combinations
Pluri- agonism targeting GLP1, GIP, Glucagon, Amylin etc... receptors represent the most promising strategies to produce satiety and promote weight loss in overweight and obese people, but the central mechanisms are unknown.
We use unbiased activity-defined circuit labelling approaches to identify and characterise the cell populations engaged in response to these drug treatments (Roth, Molecular Metabolism, 2021).
Oligodendrocytes and nutrient/metabolic sensing
We found that myelin in the median eminence, at the base of the hypothalamus, rapidly and continuously turns over, which seems to be unique to this brain region (Buller, 2023).
The median eminence is a key nutrient and metabolic sensing area of the brain, which regulates how appetite-regulating neurons access circulating nutrients and hormones, and is also involved in the control of neuroendocrine outputs.
Myelin turnover here is sensitive to acute and long-term signals of energy availability. Oligodendrocyte plasticity in the median eminence also directly influences the local extracellular matrix (Kohnke, Cell Reports, 2021), emerging as an important regulator of local nutrient sensing.
Ongoing work in the lab continues to investigate these sensing properties and their functional consequences .
 |
|---|
 |
 |
 |